
What needs to be rewritten is: Editor | Kaixia
Chemistry, starting from the ancient alchemy of "equivalent exchange", has always been a discipline that studies and controls the relationship between substances. Interacting disciplines. By continually unlocking and exploiting new chemical reactions, many new materials have been developed. These new materials not only bring convenience to people's lives, but also improve energy efficiency and promote sustainable development
A basic chemical reaction consists of reactants, transition states (TS), and products . Transition states are crucial 3D structures in chemistry and are widely used to understand chemical reaction mechanisms, estimate reaction energy barriers, and explore vast reaction networks. However, due to the extremely short time (femtosecond order) in which they exist during the reaction, it is almost impossible to isolate and characterize the transition state experimentally.
Rewritten content: Usually, people will use quantum chemical calculation methods to determine the transition state between known reactants and products by repeatedly solving the Schrödinger equation. However, this computational method is very expensive and notorious for its frequent failures. At the same time, this method is limited by personal experience, intuition and computational resources, and the chemical reactions that each person can explore are also limited. This limitation is particularly fatal when studying unknown complex reactions. It will cause researchers to ignore some potential reactions, thereby misjudging the reaction mechanism, thereby affecting the design of catalytic materials
In response to this problem, a group of researchers from the Massachusetts Institute of Technology (MIT) developed An alternative method based on machine learning was developed that can discover these structures in seconds. Their new model could assist chemists in exploring and designing new reactions and catalysts to generate useful products with high added value, such as fuel compounds or pharmaceuticals. In addition, the model is capable of simulating naturally occurring chemical reactions, such as those key to the evolution of life on early Earth.
Heather Kulik, professor of chemical engineering and chemistry at MIT, pointed out that understanding the specific structure of the transition state is very important for designing catalysts or understanding how natural systems perform certain transformations
Related research work is titled "Accurate "Transition state generation with an object-aware equivariant elementary reaction diffusion model" was published in the top international journal "Nature Computational Science".
Dr. Duan Chenru from MIT is the first author of the paper. Du Yuanqi, a doctoral student from Cornell University, Jia Haojun, a doctoral student from MIT, and Professor Heather Kulik from MIT are Co-author of the paper. Original link: [https://rdcu.be/dtGSF]
Please click the following link to view the paper: https://www.nature.com/articles/s43588- 023-00563-7
MIT News also reported on this research

Reporting link: https://news.mit. edu/2023/computational-model-captures-elusive-transition-states-1215
What needs to be rewritten is: Theoretical difficulties
Currently, chemists can Transition states are calculated using quantum chemical calculations based on density functional theory. However, this method requires a lot of computing resources, and it takes hours or even days to complete the calculation of a transition state
In order to solve the problem of long calculation time, some researchers have recently begun to try to use machine learning models to discover Transition state structure. However, almost all models developed to date require that the two reactants be modeled as a whole, with the reactants maintaining a specific geometric configuration relative to each other. Any other possible configuration will be mistaken by the machine learning model as a new reaction
Dr. Duan Chenru said that if the reactant molecules are rotated, in principle, they can still experience the same before and after rotation chemical reaction. Just like when we talk about electrolyzing water, we only say that water is converted into oxygen and hydrogen under specific conditions, without describing the relative geometric positions of these molecules. But in traditional machine learning methods, the model will treat the reactions of reactants and products at different geometric positions as two different reactions. This makes machine learning training more difficult and accuracy will decrease
The diffusion model is a generative model that has been widely used in image processing. Recently, diffusion models have also been used to generate 3D molecular and protein structures, protein-ligand docking and structure-based drug design. In these applications, diffusion models use 3D special Euclidean group (SE(3)) graph neural networks (GNNs) to preserve the alignment, translational and rotational symmetries of molecules. However, elementary reactions consist of reactants, transition states, and products and follow the "object-aware" SE(3) symmetry. This is because the interaction between the three objects in the elementary reaction is not carried out in the 3D Euclidean space, but is a causal connection on the higher-dimensional electronic potential energy surface. Therefore, the existing diffusion model based on SE(3) GNN may have problems due to the destruction of symmetry
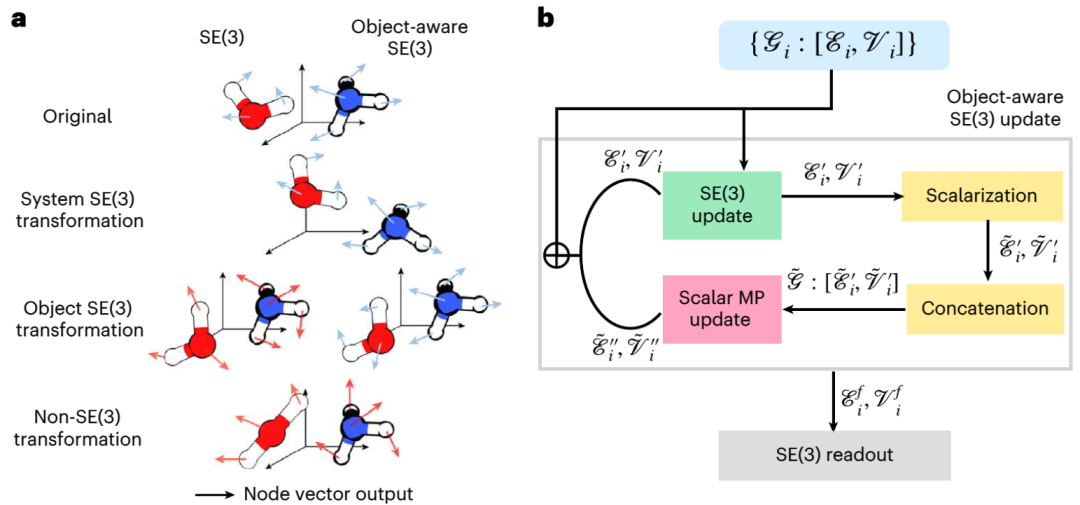
Illustration: "Object Perception" SE(3) etc. Variation and its implementation based on SE(3) equivariant GNN. (Source: paper)
Solution
The MIT team developed a new method called "OA-ReactDiff" based on the above problems. The team adapted the SE(3) equivariant GNN to "object-aware" simulation, that is, maintaining the SE(3) equivariance of individual objects while maintaining their independent interactions in Euclidean space
Dr. Duan Chenru said that the diffusion model is part of the field of generative artificial intelligence, which captures the transformation process between simple distributions and complex distributions through random processes. Once the model learns the basic distribution of how these three structures coexist, we can give it new reactants and products and it will try to generate transition state structures that correspond to those reactants and products
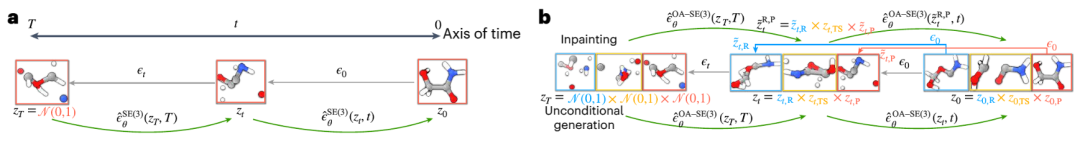
Illustration: Overview of the Equivariant Diffusion Model (EDM) used to generate samples of molecular systems. (Source: paper)
In the study, researchers used quantum computing methods to obtain the structures of reactants, transition states and products of 9,000 different chemical reactions in the training set. And also tested about 1,000 previously unseen reactions, requiring the generation of 40 possible structures for each transition state
In the calculation process, a "recommendation model" was introduced to predict which transition The highest confidence level. On this basis, further combined with uncertainty estimates, the researchers only performed quantum chemical calculations on the 14% of reactions with the highest model uncertainty, successfully achieving an average absolute error of 2.6 kcal/mol. This allows for results within an order of magnitude error when estimating reaction rates at 300°C using OA-ReactDiff. Compared with the transition state structure obtained by quantum chemical calculations, the root mean square error (RMSD) of the structure generated by OA-ReactDiff is in the range of 0.06 Angstroms (6 thousandths of a nanometer), an error magnitude that is almost indistinguishable to the naked eye
What is even more gratifying is that OA-ReactDiff only takes 6 seconds to generate a transition state structure, which is at least 1000 times faster than quantum chemical calculations. As a result, the algorithm successfully achieves extremely high accuracy and rapidity in calculating TS structures and reaction energy barriers.
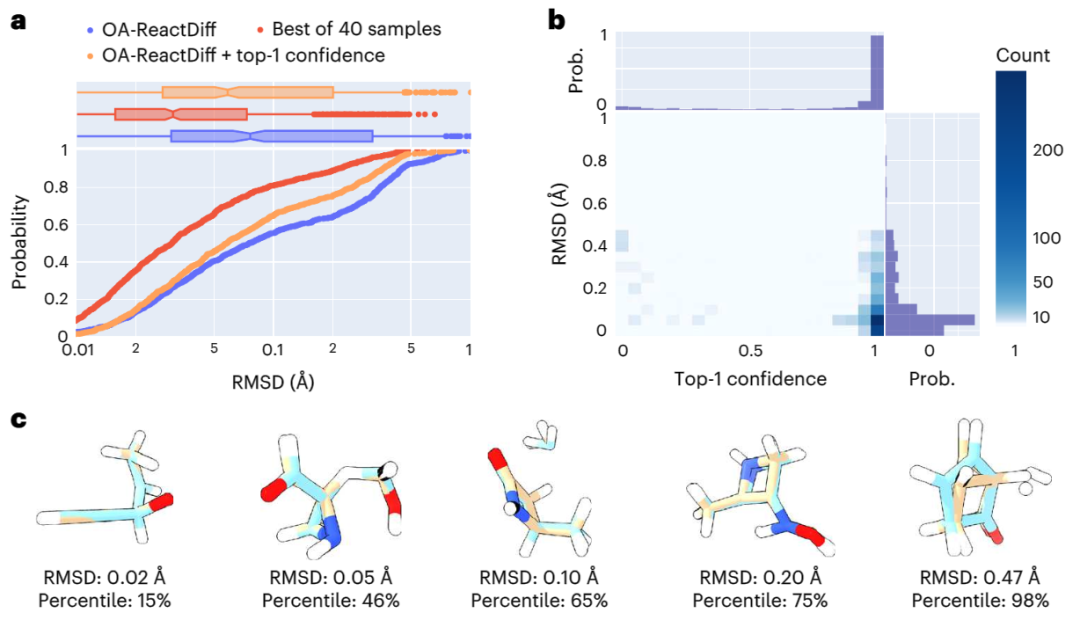
Illustration: Evaluating the structural similarity between the TS structure generated by OA-ReactDiff and the real TS structure. (Source: paper)
Professor Kulik also lamented, "It was difficult for us to imagine that thousands of transition states can be generated in one thought."
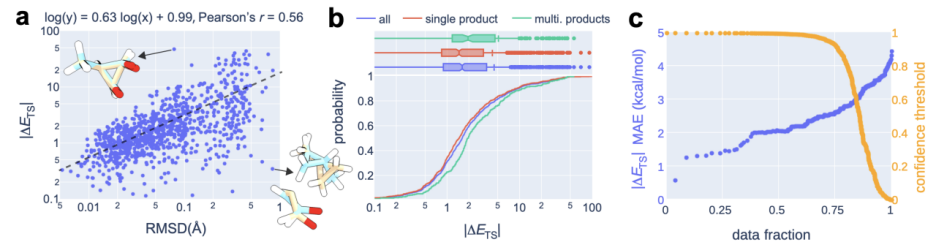
The content that needs to be rewritten is: Illustration: OA-ReactDiff plus recommends the energy performance of the TS structure. (Source: Paper)
Future expectations are expectations and hopes for the future. It is a person's vision for his or her future development and growth. Everyone has their own future expectations, which can be to achieve personal goals, pursue career success, build a happy family, or make positive contributions to society, etc. No matter what the future expectations are, it is the motivation and direction for people to work hard. Through hard work and persistence, we can gradually realize our future expectations and make our lives better and more fulfilling
What needs to be rewritten is: This research is the first to use 3D in chemical reactions Diffusion model. The significance of this work cannot be ignored, although the researchers only studied compounds with smaller numbers of atoms (
Professor Kulik pointed out: "Even when faced with larger systems or even enzyme-catalyzed systems, it is still possible to obtain different ways in which the atoms are most likely to rearrange."
The researchers now plan to extend their model by adding other components, such as catalysts. Leveraging the randomness of generative AI, OA-ReactDiff can explore unexpected chemical reactions. This feature complements the existing chemistry-based intuitive reaction exploration framework, helps establish a more complete chemical reaction network, and assists in the research and development of new catalytic materials. Research in this area can help them accelerate the discovery of new catalysts for specific reactions. Additionally, their proposed algorithm could be useful for developing new processes for drugs, fuels, or other useful compounds, especially when the synthesis involves many chemical steps.
Dr. Duan Chenru pointed out that in the past, all these calculations were performed using quantum chemistry methods, but now we can replace quantum chemistry with faster generation models
The researchers also pointed out that chemical reactions It is the core of chemical research. In addition to catalyst design that is biased toward industrial applications, OA-ReactDiff also has many interesting potential applications, such as exploring gas interactions that may occur on other planets, simulating reaction processes during the evolution of early life on Earth, etc.
The above is the detailed content of AI alchemy revolutionizes chemistry: MIT scholars use generative AI to generate new chemical reactions in six seconds. For more information, please follow other related articles on the PHP Chinese website!
 What to do if win8wifi connection is not available
What to do if win8wifi connection is not available
 Comparative analysis of iqooneo8 and iqooneo9
Comparative analysis of iqooneo8 and iqooneo9
 How to use fit function in Python
How to use fit function in Python
 Solid state drive data recovery
Solid state drive data recovery
 Change word background color to white
Change word background color to white
 Google earth cannot connect to the server solution
Google earth cannot connect to the server solution
 What are the reasons why a mobile phone has an empty number?
What are the reasons why a mobile phone has an empty number?
 css beyond display...
css beyond display...




