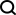Technology peripherals
Technology peripherals
 AI
AI
 SOTA performance, Xiamen multi-modal protein-ligand affinity prediction AI method, combines molecular surface information for the first time
SOTA performance, Xiamen multi-modal protein-ligand affinity prediction AI method, combines molecular surface information for the first time
SOTA performance, Xiamen multi-modal protein-ligand affinity prediction AI method, combines molecular surface information for the first time

Editor | KX
In the field of drug research and development, accurately and effectively predicting the binding affinity of proteins and ligands is crucial for drug screening and optimization. However, current studies do not take into account the important role of molecular surface information in protein-ligand interactions.
Based on this, researchers from Xiamen University proposed a novel multi-modal feature extraction (MFE) framework, which for the first time combines information on protein surface, 3D structure and sequence, and uses a cross-attention mechanism for different modes. Feature alignment between states.
Experimental results show that this method achieves state-of-the-art performance in predicting protein-ligand binding affinity. Furthermore, ablation studies demonstrate the effectiveness and necessity of protein surface information and multimodal feature alignment within this framework.
Related research titled "Surface-based multimodal protein–ligand binding affinity prediction" was published on "Bioinformatics" on June 21.

Protein-ligand binding affinity prediction research
As a key stage of drug discovery, predicting protein-ligand binding affinity has been extensively studied for a long time, which is crucial for efficient and accurate drug screening.
Traditional computer-aided drug discovery tools use scoring functions (SF) to roughly estimate protein-ligand binding affinity, but with low accuracy. Molecular dynamics simulation methods can provide more accurate binding affinity estimates but are often costly and time-consuming.
With the development of computing technology and the increasing abundance of large-scale biological data, deep learning-based methods have shown great potential in the field of protein-ligand binding affinity prediction.
However, current research mainly utilizes sequence- or structure-based representations to predict protein-ligand binding affinity, and there are relatively few studies on protein surface information that is crucial for protein-ligand interactions.
A molecular surface is a high-level representation of a protein's structure, which exhibits characteristic chemical and geometric patterns that serve as fingerprints of the protein's interaction patterns with other biomolecules. Therefore, some studies began to use protein surface information to predict protein-ligand binding affinity.
But existing methods mainly focus on single-modal data and ignore the multi-modal information of proteins. Furthermore, when processing multimodal information of proteins, traditional methods usually connect features from different modalities in a direct manner without considering the heterogeneity between them, which results in the inability to effectively exploit the complementarity between modalities. .
Novel multi-modal feature extraction framework
Here, researchers propose a novel multi-modal feature extraction (MFE) framework that for the first time combines information from protein surface, 3D structure and sequence .
Specifically, the study designed two main components: protein feature extraction module and multi-modal feature comparison module.
The protein feature extraction module is used to extract initial embeddings from protein surface, structure and sequence information.
In the multi-modal feature comparison module, the cross-attention mechanism is used to achieve feature comparison between protein structure, sequence embedding and surface embedding to obtain a unified and information-rich feature embedding.
Compared with current state-of-the-art methods, the proposed framework achieves the best results on the protein-ligand binding affinity prediction task.
SOTA Performance
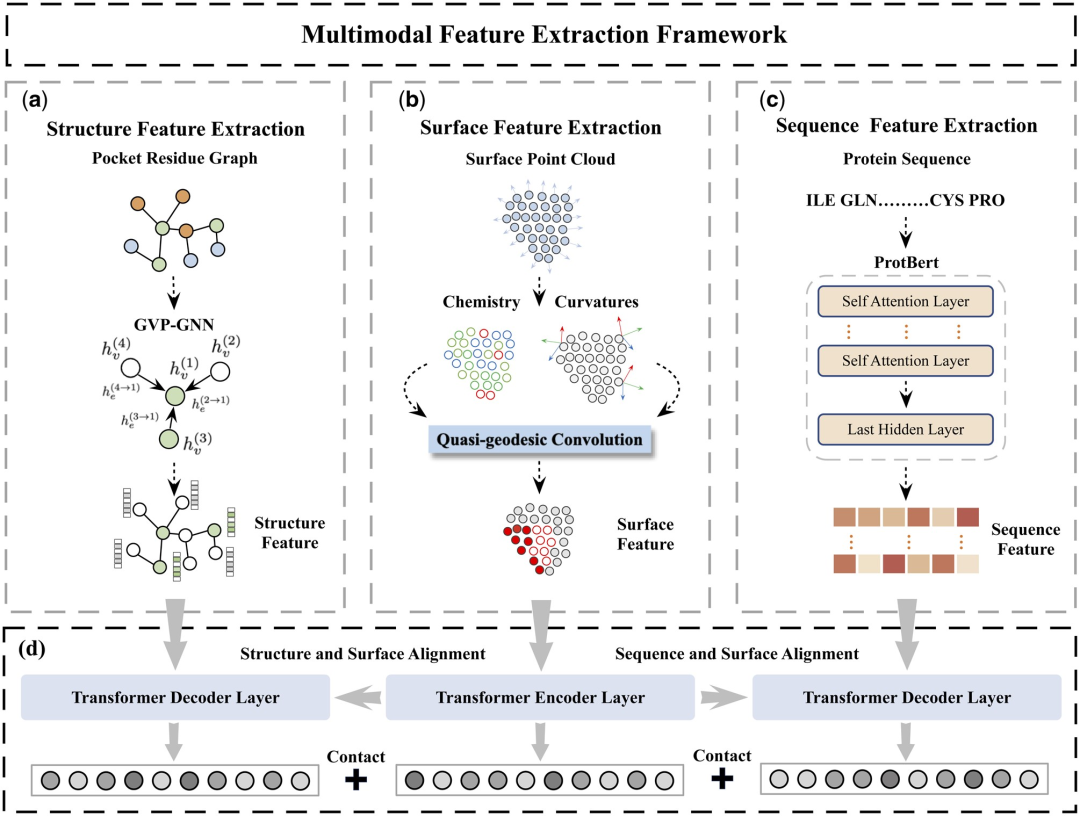
Table 1 shows the results of MFE and other baseline models on the protein-ligand binding affinity prediction task. All models used the same training and validation set partitioning method and were tested on the PDBbind core set (version 2016). It can be found that the MFE method achieves SOTA performance compared to all baselines.
Ablation Study
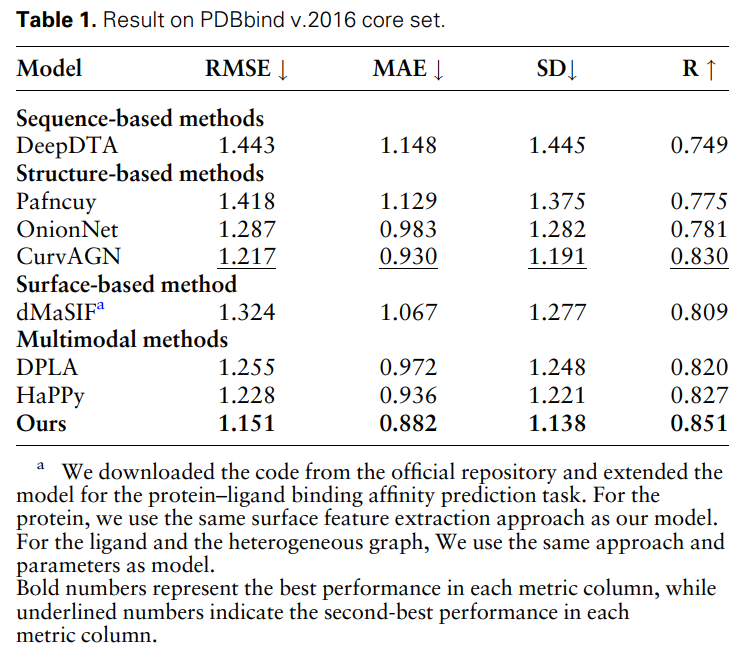
To further prove the effectiveness and necessity of different modal features and feature comparisons, the researchers conducted the following ablation studies: W/O protein surface information, W/O protein structure information , w/o protein sequence information and featureless alignments. The results are shown in Table 2 and Figure 2.
Figure 2: Ablation study results. (Source: paper)
The results show that when surface information is removed, the performance drops significantly, indicating that surface information plays a crucial role in the model. Likewise, excluding either structural or sequence information results in performance degradation, while elimination of sequence information results in a more pronounced degradation. This is because sequence information contains global information about the protein, which is crucial for the model to fully understand the protein.
In addition, without feature comparison, the performance of the model will decrease. This emphasizes the importance of feature comparison in processing multi-modal data, as it helps reduce the heterogeneity between different modal features, thereby improving the model's ability to effectively integrate different modal features.
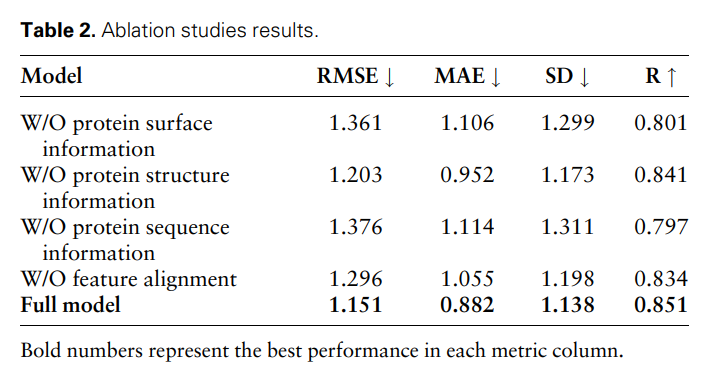
Hyperparameter analysis
In order to study the impact of different hyperparameters on model performance, the researchers conducted the following three experiments: (i) MFE-A-6: only use 6 basic atom types to represent Chemical properties of the surface, including hydrogen, carbon, nitrogen, oxygen, phosphorus, and sulfur; (ii) MFE-P-256: Only the 256 surface points closest to the ligand center are selected as the protein pocket surface; (iii) MFE-P -1024: Select the 1024 surface points closest to the ligand center as the protein pocket surface.
Figure 3 shows the results of three different hyperparameter selection methods on the protein-ligand binding affinity prediction task.
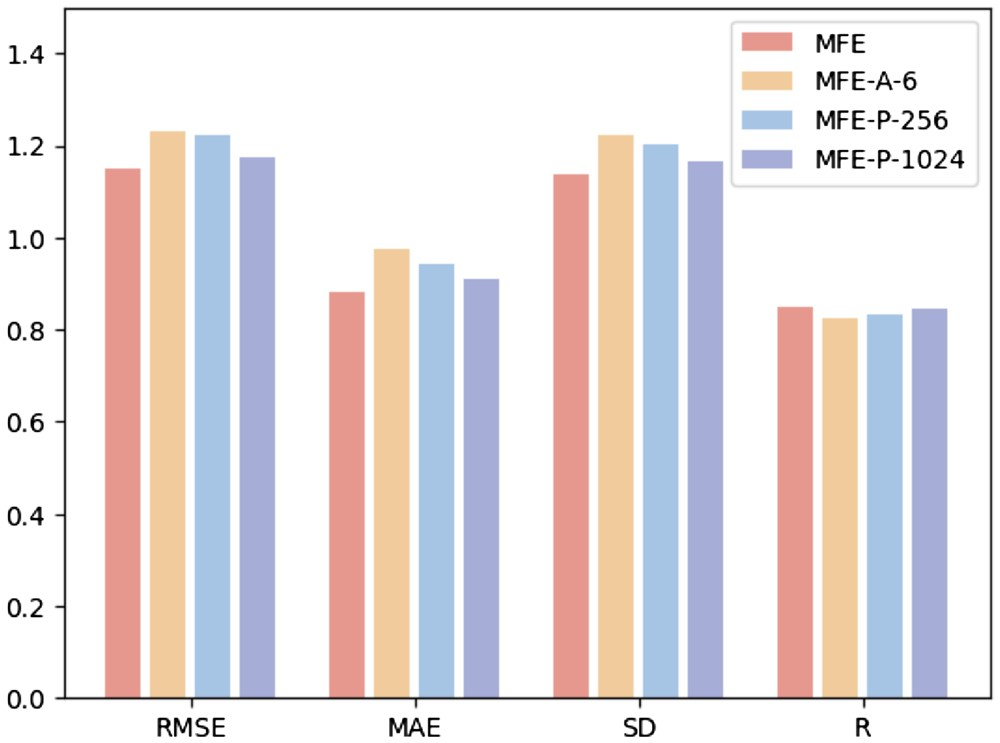
Feature Alignment Analysis and Visualization
In order to deeply study the impact of feature alignment on model performance, the researchers used principal component analysis (PCA) to perform dimensionality reduction and summation of protein surface, structural and sequence features in the test set Visual analysis. This approach aims to determine whether feature alignment can mitigate heterogeneity between multimodal embeddings.
Research found that feature alignment significantly enhanced the consistency between protein surface, structure and sequence embedding. This is due to the optimization of multi-modal feature interactions in Transformer through the attention mechanism, which calculates attention weights between different features. This enhances the model's ability to capture key information, allowing data from different modalities to be more closely clustered in feature space, thereby reducing noise and errors in the model's identification of protein-ligand interactions.
Finally, the researchers concluded, “In summary, by studying the surface of proteins, we can gain a deeper understanding of how proteins interact with other biomolecules. In future work, we will explore protein surfaces more thoroughly to reveal Their wider application in bioinformatics"
Note: The cover comes from the Internet
.The above is the detailed content of SOTA performance, Xiamen multi-modal protein-ligand affinity prediction AI method, combines molecular surface information for the first time. For more information, please follow other related articles on the PHP Chinese website!

Hot AI Tools

Undresser.AI Undress
AI-powered app for creating realistic nude photos

AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover

AI Hentai Generator
Generate AI Hentai for free.

Hot Article

Hot Tools

Notepad++7.3.1
Easy-to-use and free code editor

SublimeText3 Chinese version
Chinese version, very easy to use

Zend Studio 13.0.1
Powerful PHP integrated development environment

Dreamweaver CS6
Visual web development tools

SublimeText3 Mac version
God-level code editing software (SublimeText3)

Hot Topics
 1381
1381
 52
52
 Bytedance Cutting launches SVIP super membership: 499 yuan for continuous annual subscription, providing a variety of AI functions
Jun 28, 2024 am 03:51 AM
Bytedance Cutting launches SVIP super membership: 499 yuan for continuous annual subscription, providing a variety of AI functions
Jun 28, 2024 am 03:51 AM
This site reported on June 27 that Jianying is a video editing software developed by FaceMeng Technology, a subsidiary of ByteDance. It relies on the Douyin platform and basically produces short video content for users of the platform. It is compatible with iOS, Android, and Windows. , MacOS and other operating systems. Jianying officially announced the upgrade of its membership system and launched a new SVIP, which includes a variety of AI black technologies, such as intelligent translation, intelligent highlighting, intelligent packaging, digital human synthesis, etc. In terms of price, the monthly fee for clipping SVIP is 79 yuan, the annual fee is 599 yuan (note on this site: equivalent to 49.9 yuan per month), the continuous monthly subscription is 59 yuan per month, and the continuous annual subscription is 499 yuan per year (equivalent to 41.6 yuan per month) . In addition, the cut official also stated that in order to improve the user experience, those who have subscribed to the original VIP
 Breaking through the boundaries of traditional defect detection, 'Defect Spectrum' achieves ultra-high-precision and rich semantic industrial defect detection for the first time.
Jul 26, 2024 pm 05:38 PM
Breaking through the boundaries of traditional defect detection, 'Defect Spectrum' achieves ultra-high-precision and rich semantic industrial defect detection for the first time.
Jul 26, 2024 pm 05:38 PM
In modern manufacturing, accurate defect detection is not only the key to ensuring product quality, but also the core of improving production efficiency. However, existing defect detection datasets often lack the accuracy and semantic richness required for practical applications, resulting in models unable to identify specific defect categories or locations. In order to solve this problem, a top research team composed of Hong Kong University of Science and Technology Guangzhou and Simou Technology innovatively developed the "DefectSpectrum" data set, which provides detailed and semantically rich large-scale annotation of industrial defects. As shown in Table 1, compared with other industrial data sets, the "DefectSpectrum" data set provides the most defect annotations (5438 defect samples) and the most detailed defect classification (125 defect categories
 NVIDIA dialogue model ChatQA has evolved to version 2.0, with the context length mentioned at 128K
Jul 26, 2024 am 08:40 AM
NVIDIA dialogue model ChatQA has evolved to version 2.0, with the context length mentioned at 128K
Jul 26, 2024 am 08:40 AM
The open LLM community is an era when a hundred flowers bloom and compete. You can see Llama-3-70B-Instruct, QWen2-72B-Instruct, Nemotron-4-340B-Instruct, Mixtral-8x22BInstruct-v0.1 and many other excellent performers. Model. However, compared with proprietary large models represented by GPT-4-Turbo, open models still have significant gaps in many fields. In addition to general models, some open models that specialize in key areas have been developed, such as DeepSeek-Coder-V2 for programming and mathematics, and InternVL for visual-language tasks.
 Training with millions of crystal data to solve the crystallographic phase problem, the deep learning method PhAI is published in Science
Aug 08, 2024 pm 09:22 PM
Training with millions of crystal data to solve the crystallographic phase problem, the deep learning method PhAI is published in Science
Aug 08, 2024 pm 09:22 PM
Editor |KX To this day, the structural detail and precision determined by crystallography, from simple metals to large membrane proteins, are unmatched by any other method. However, the biggest challenge, the so-called phase problem, remains retrieving phase information from experimentally determined amplitudes. Researchers at the University of Copenhagen in Denmark have developed a deep learning method called PhAI to solve crystal phase problems. A deep learning neural network trained using millions of artificial crystal structures and their corresponding synthetic diffraction data can generate accurate electron density maps. The study shows that this deep learning-based ab initio structural solution method can solve the phase problem at a resolution of only 2 Angstroms, which is equivalent to only 10% to 20% of the data available at atomic resolution, while traditional ab initio Calculation
 Google AI won the IMO Mathematical Olympiad silver medal, the mathematical reasoning model AlphaProof was launched, and reinforcement learning is so back
Jul 26, 2024 pm 02:40 PM
Google AI won the IMO Mathematical Olympiad silver medal, the mathematical reasoning model AlphaProof was launched, and reinforcement learning is so back
Jul 26, 2024 pm 02:40 PM
For AI, Mathematical Olympiad is no longer a problem. On Thursday, Google DeepMind's artificial intelligence completed a feat: using AI to solve the real question of this year's International Mathematical Olympiad IMO, and it was just one step away from winning the gold medal. The IMO competition that just ended last week had six questions involving algebra, combinatorics, geometry and number theory. The hybrid AI system proposed by Google got four questions right and scored 28 points, reaching the silver medal level. Earlier this month, UCLA tenured professor Terence Tao had just promoted the AI Mathematical Olympiad (AIMO Progress Award) with a million-dollar prize. Unexpectedly, the level of AI problem solving had improved to this level before July. Do the questions simultaneously on IMO. The most difficult thing to do correctly is IMO, which has the longest history, the largest scale, and the most negative
 Nature's point of view: The testing of artificial intelligence in medicine is in chaos. What should be done?
Aug 22, 2024 pm 04:37 PM
Nature's point of view: The testing of artificial intelligence in medicine is in chaos. What should be done?
Aug 22, 2024 pm 04:37 PM
Editor | ScienceAI Based on limited clinical data, hundreds of medical algorithms have been approved. Scientists are debating who should test the tools and how best to do so. Devin Singh witnessed a pediatric patient in the emergency room suffer cardiac arrest while waiting for treatment for a long time, which prompted him to explore the application of AI to shorten wait times. Using triage data from SickKids emergency rooms, Singh and colleagues built a series of AI models that provide potential diagnoses and recommend tests. One study showed that these models can speed up doctor visits by 22.3%, speeding up the processing of results by nearly 3 hours per patient requiring a medical test. However, the success of artificial intelligence algorithms in research only verifies this
 To provide a new scientific and complex question answering benchmark and evaluation system for large models, UNSW, Argonne, University of Chicago and other institutions jointly launched the SciQAG framework
Jul 25, 2024 am 06:42 AM
To provide a new scientific and complex question answering benchmark and evaluation system for large models, UNSW, Argonne, University of Chicago and other institutions jointly launched the SciQAG framework
Jul 25, 2024 am 06:42 AM
Editor |ScienceAI Question Answering (QA) data set plays a vital role in promoting natural language processing (NLP) research. High-quality QA data sets can not only be used to fine-tune models, but also effectively evaluate the capabilities of large language models (LLM), especially the ability to understand and reason about scientific knowledge. Although there are currently many scientific QA data sets covering medicine, chemistry, biology and other fields, these data sets still have some shortcomings. First, the data form is relatively simple, most of which are multiple-choice questions. They are easy to evaluate, but limit the model's answer selection range and cannot fully test the model's ability to answer scientific questions. In contrast, open-ended Q&A
 PRO | Why are large models based on MoE more worthy of attention?
Aug 07, 2024 pm 07:08 PM
PRO | Why are large models based on MoE more worthy of attention?
Aug 07, 2024 pm 07:08 PM
In 2023, almost every field of AI is evolving at an unprecedented speed. At the same time, AI is constantly pushing the technological boundaries of key tracks such as embodied intelligence and autonomous driving. Under the multi-modal trend, will the situation of Transformer as the mainstream architecture of AI large models be shaken? Why has exploring large models based on MoE (Mixed of Experts) architecture become a new trend in the industry? Can Large Vision Models (LVM) become a new breakthrough in general vision? ...From the 2023 PRO member newsletter of this site released in the past six months, we have selected 10 special interpretations that provide in-depth analysis of technological trends and industrial changes in the above fields to help you achieve your goals in the new year. be prepared. This interpretation comes from Week50 2023